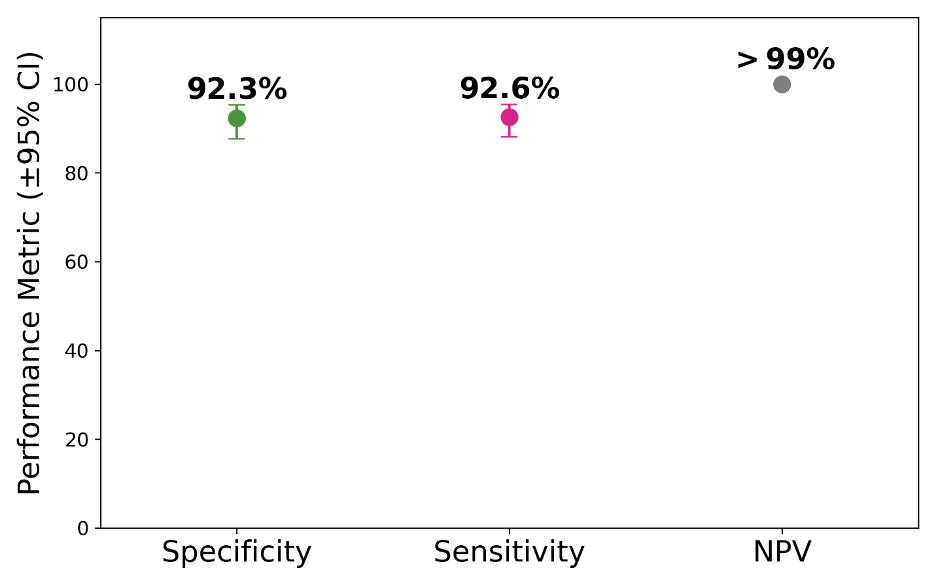Improve Patient Confidence Without Increasing False Positives
High sensitivity. High specificity. Less anxiety for you and your patients.
>99% NPV demonstrates Certitude's ability to avoid unnecessary imaging and biopsies.

 Certitude is a simple, lab-based blood test designed to detect early breast cancer–associated proteins.
Certitude is a simple, lab-based blood test designed to detect early breast cancer–associated proteins. Helps you further risk-stratify your patients with accuracy and confidence while reducing unnecessary imaging, false positives, and avoidable biopsies.
Helps you further risk-stratify your patients with accuracy and confidence while reducing unnecessary imaging, false positives, and avoidable biopsies.
How does Certitude Work?
The Tech Behind the Test
Sample Processing
Blood Sample
Protein Isolation
Peptide Generation
Protein Analysis
Data Analysis
Protein Identification
Machine Learning
Cancer Proteins Detected
Certitude employs proprietary proteomic analysis to map patterns of circulating protein expression that are distinctly correlated with breast malignancy. These highly specific biomarkers are processed by sophisticated machine-learning algorithms — trained on extensive, clinically validated datasets — to deliver accurate, reproducible results.
Certitude Performance
The following performance metrics demonstrate Certitude’s effectiveness across key diagnostic dimensions, including NPV, sensitivity/specificity, and consistency across stage.
Learn MoreCertitude is an Innovative, CLIA-Certified Blood Test Developed by Astrin Biosciences.
It uses advanced proteomic analysis and machine learning to detect protein patterns associated with breast cancer.
Elevate Early Detection
Identify molecular signatures of breast cancer even when imaging is equivocal or inconclusive
Strengthen Clinical Decision-Making
Integrate objective proteomic data with imaging findings and patient risk profiles to guide patients in their screening journey
Maximize Patient Compliance
A familiar, non-invasive blood draw minimizes anxiety and can be conveniently incorporated into any routine office visit
Enable Shared Decision-Making
Provide women with dense breasts or elevated risk a clear path toward more informed and proactive screening next steps
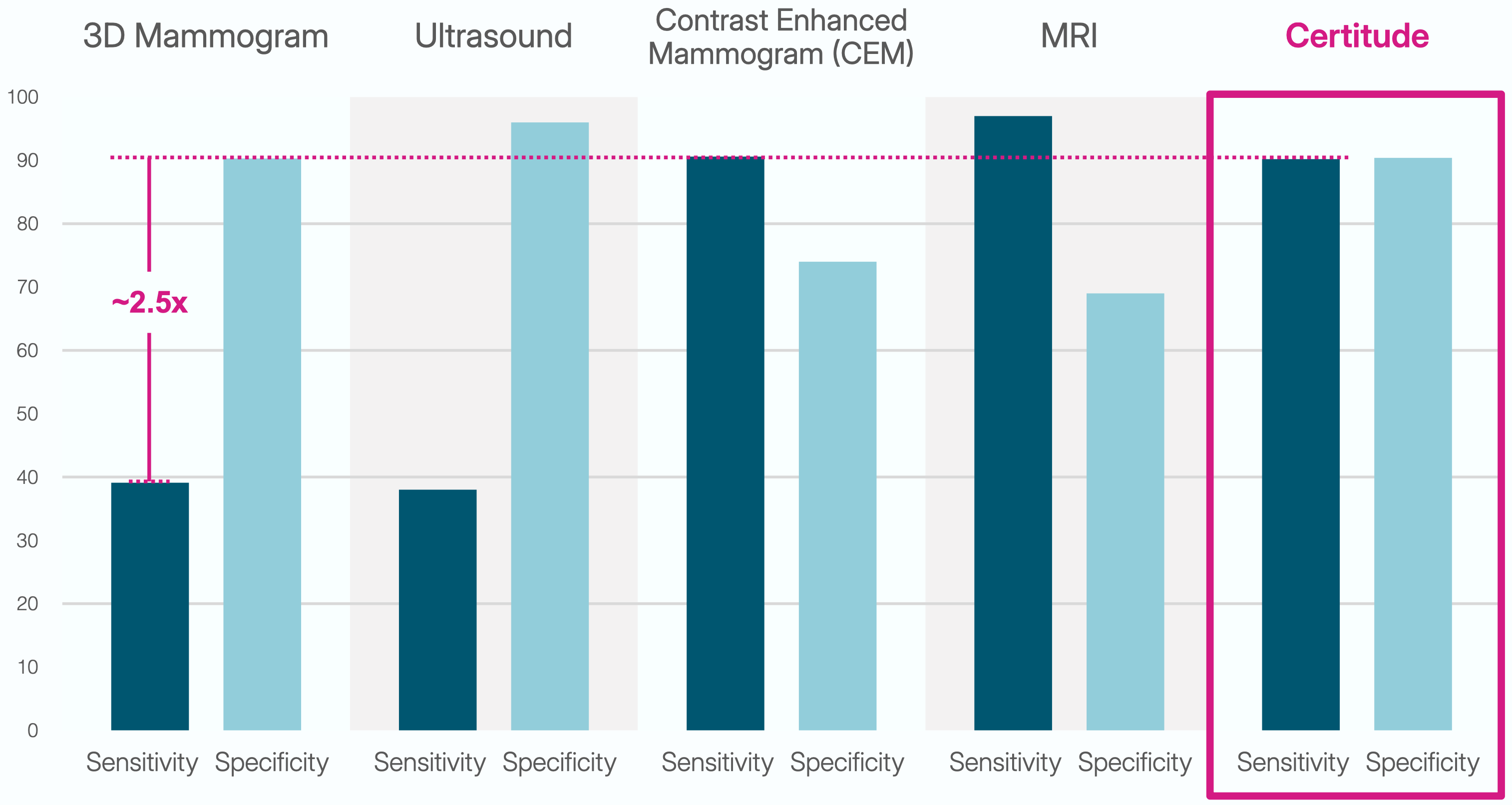
Sensitivity & Specificity at a Glance
Compare published sensitivity and specificity across 3D mammography, ultrasound, CEM, MRI, and Certitude for women with dense breasts. These data do not come from a head-to-head study but provide a comparative side-to-side view.
To help contextualize Certitude’s clinical utility, the table below compares its performance with mammography and MRI.
| Mammography | MRI | Certitude Blood Test | |
|---|---|---|---|
| Detection | Limited visibility in dense tissue | High sensitivity, frequent false positives | High sensitivity, high specificity with 99% NPV |
| Patient Experience | Compression, radiation exposure | Time-intensive, expensive, limited access | Simple, standard blood draw |
| Accessibility | Widely available | Specialist centers only | Broadly accessible via standard labs or mobile phlebotomy |
| Use Case | Primary screening | Follow-up/High-risk imaging | Adjunctive screening to avoid unnecessary imaging and treatment |
Just Blood. No Sweat. No Tears.
Certitude integrates effortlessly into any clinical setting—requiring no new equipment or complex logistical changes.
Workflow Process
Register your practice via the secure Certitude provider portal.
Negative Result: A negative result indicates the test did not find breast cancer-associated signals.
Positive Result: A positive result indicates signals associated with breast cancer were detected. It does not necessarily mean cancer. If a patient receives a positive test result, next steps are typically to administer a breast MRI or a biopsy, if needed, as a confirmatory test but will be determined as per the treating provider's discretion and judgement.


Coverage & Support
 We are actively working on establishing insurance coverage for the Certitude test.
We are actively working on establishing insurance coverage for the Certitude test. The Certitude test is available via self-pay. Patients will have to cover the out-of-pocket cost.
The Certitude test is available via self-pay. Patients will have to cover the out-of-pocket cost. Patients may be able to use pretax dollars in their flexible spending account (FSA) or health savings account (HSA) to pay for the Certitude test.
Patients may be able to use pretax dollars in their flexible spending account (FSA) or health savings account (HSA) to pay for the Certitude test.